Transcranial Magnetic Stimulation
Rejuvenate your mental health with Transcranial Magnetic Stimulation (TMS). Our non-invasive, drug-free treatment offers a clinically proven solution for treatment-resistant depression.
Safe and effective alternative for patients not responding to medication
FDA-approved with 49% response rate and 32% remission rate
No anesthesia or systemic side effects, covered by insurance
“TMS has changed my life.”
TMS has changed my life. I’ve been able to reduce my medications, my depression is gone, and it’s the only thing that’s worked for my insomnia.
-Mercedes K.
Transcranial magnetic stimulation – also known as TMS – uses focused magnetic pulses to improve brain activity. TMS induces changes in activity of nerve cells by applying rapidly changing or repetitive magnetic fields. Thus, TMS is also called repetitive TMS (rTMS).
Transcranial magnetic stimulation is a clinically meaningful and effective treatment of depression (major depressive disorder) (Lefaucheur et al., 2014; McClintock et al., 2017). The treatment is non-invasive, and does not require anesthesia. TMS is a drug-free alternative to antidepressant medication, and is offered to patients either not responding to their medication or who cannot tolerate the side effects.
The largest clinical trial using rTMS for treatment of depression shows that 49% of patients who previously failed to receive improvement from prior antidepressant medication responded to treatment, and 32% received remission, meaning they were no longer clinically depressed (Blumberger et al., 2018). TMS has been marketed and approved for this purpose for almost a decade in both Europe and the USA and is covered by insurance (McClintock et al., 2017).
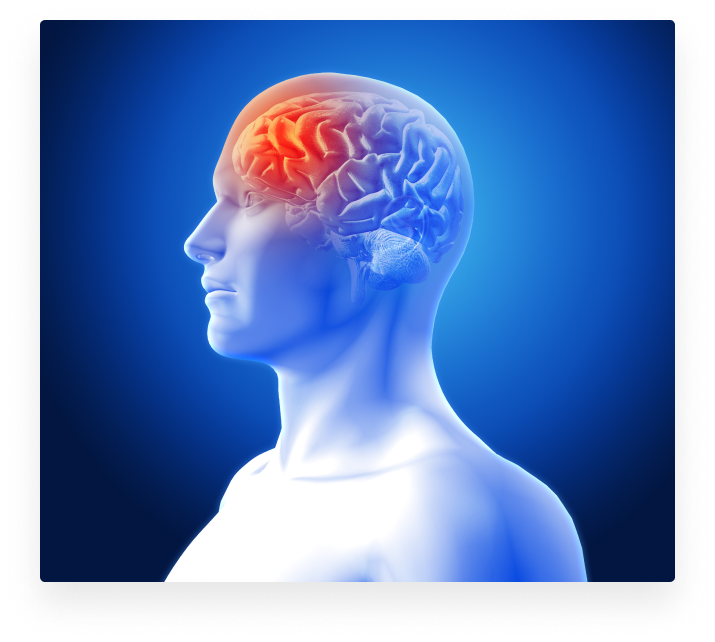

Why is TMS effective in treating depression?
The most common symptoms of depression is the presence of empty, sad or irritable mood in combination with both cognitive and somatic changes that can significantly affect the individual’s capacity to function (Downar, Blumberger, Daskalakis, 2016). These behavioral and functional consequences of depression are due to alterations in brain activity. In depression, a whole distributed network of brain areas is affected.
The cardinal idea of applying TMS for treatment of depression is to precisely target the areas of the brain involved in treatment-resistant depression. The part of the brain that is being stimulated is located to the left side of the brain, specifically the dorso-lateral prefrontal cortex, or in short Left-DLPFC. This cortical area is the prime target for CE and FDA approved TMS treatment, as it is a focal point connecting all the different brain areas that are involved in the pathology of depression (Anderson, Hoy, Daskalakis, & Fitzgerald, 2016; Tik et al., 2017). Insurance will often cover TMS if the patient is treatment resistant, meaning that anti-depressants from two different medication classes have failed to resolve symptoms. If insurance denies TMS, we offer TMS/ketamine combination therapy for patients our providers approve, so they’ll get better faster.
Depression treatment without systemic side effects
The Left-DLPFC is responsible for:
Coordinating and adjusting complex behavior
Controlling impulses
Displaying appropriate emotional reactions
Manifesting personality
Focusing attention
Considering and prioritizing competing and simultaneous information
Ignoring external distractions
Complex planning
Assessing options and choosing a course of behavior
Deferring certain immediate gratifications in order to obtain greater long-term benefits
The left half of pre-frontal cortex is involved in establishing positive feelings
The right half is involved in establishing negative feelings
Thus, stimulating at the focal point and modulating its activity will consequently modulate the activity in other areas of the brain, and thereby transcranial magnetic stimulation is selectively improving overall brain activity (Chen et al., 2013; Liston et al., 2014). Or in other words, TMS enhances entire brain networks, alleviating depression and the behavioral and cognitive symptoms, without any of the systemic side effects often associated with medication.

FAQs
TMS has been shown to produce changes in neuronal activity in regions of the brain implicated in mood regulation, such as the prefrontal cortex. As each magnetic pulse passes through the skull and into the brain, this induces brief activity of brain cells underlying the treatment coil. The frequency of pulse delivery also influences whether brain activity is increased or decreased by a session of TMS. Recent studies also suggest that stimulation over the left and right sides of the brain can have opposite effects on mood regulation (depression vs anxiety/OCD).
Antidepressant medications and psychotherapy are the first line treatments for major depression. These treatments, however, do not work for all patients. In these instances, TMS might be used as an alternative treatment, or to augment antidepressant medications, ketamine/Spravato, or psychotherapy. Patients who have failed to achieve an adequate response from at least 2 to 3 antidepressants, or who are unable to tolerate medications, might consider TMS therapy.
Because TMS uses magnetic pulses, before beginning a treatment, patients are asked to remove any magnetic-sensitive objects (such as jewelry). TMS produces a loud clicking sound with each pulse, much like an MRI machine. Patients are seated during each session of TMS. During the first rTMS session, several measurements are made to ensure that the TMS coil will be properly positioned over the patient’s head. Once this is done, the TMS coil is suspended over the patient’s scalp. We then measure the patient’s motor threshold, by administering several brief pulses. The motor threshold is the minimum amount of power necessary to make the patient’s thumb twitch, and varies from individual to individual. Measuring the motor threshold helps the physician personalize the treatment settings and determine the amount of energy required to stimulate brain cells. Once the motor threshold is determined, the coil is then brought forward so that it rests above the front region of the patient’s brain. Treatment is then commenced. During the treatment, patients will hear a series of clicking sounds and will feel a tapping sensation under the treatment coil. Motor threshold is not checked at every treatment but may be reassessed if there is concern it may have changed, for example, because of a change in medication.
TMS is always prescribed by our psychiatrist here at Ascend Health Center. The treatment itself is administered by an experienced TMS technician under the supervision of the TMS physician, or by the nurse anesthetist.
The TMS technician or physician will always be present to monitor the patient during the treatment. The patient can stop a treatment at any time by asking the staff member present.
TMS therapy involves a series of treatment sessions. Treatment sessions vary in length depending on the TMS coil used and the number of pulses delivered but typically last around 1-3 minutes. Patients receive TMS up to 5 days a week. A typical course of TMS is 4 to 6 weeks. However, this can vary depending on an individual’s response to treatment.
Unlike ECT, TMS does not require any sedation or general anesthesia, so patients are fully awake and aware during the treatment. There is no “recovery time”, so patients can drive home afterwards and return to their usual activities.
TMS is well-tolerated and associated with few side-effects and only a small percentage of patients discontinue treatment because of these. The most common side-effect, which is reported in about half of patients treated with TMS, is headaches. These are mild and generally diminish over the course of the treatment. Over-the-counter pain medication can be used to treat these headaches.
About one third of patients may experience painful scalp sensations or facial twitching with TMS pulses. These too tend to diminish over the course of treatment although adjustments can be made immediately in coil positioning and stimulation settings to reduce discomfort.
Some patients may still complain of hearing problems immediately following treatment. No evidence suggests these effects are permanent, and earplugs can be worn during the treatment.
TMS has not been associated with many of the side-effects caused by antidepressant medications, such as gastrointestinal upset, dry mouth, sexual dysfunction, weight gain, or sedation.
The most serious risk of TMS is seizures. However, the risk of a seizure is exceedingly low. At Ascend, we follow up-to-date safety guidelines that are designed to minimize the risk of seizures. While TMS is a safe procedure, it is important to point out that because it is a new treatment (first developed in the 1980’s), there may be unforeseeable risks that are not currently recognized.
Patients with any type of non-removable metal in their heads (with the exception of braces or dental fillings), should not receive rTMS. Failure to follow this rule could cause the object to heat up, move, or malfunction, and result in serious injury or death. The following is a list of metal implants that can prevent a patient from receiving rTMS:
- Aneurysm clips or coils
- Stents in the neck or brain
- Deep brain stimulators
- Electrodes to monitor brain activity
- Metallic implants in your ears and eyes
- Shrapnel or bullet fragments in or near the head
- Facial tattoos with metallic or magnetic-sensitive ink
- Other metal devices or object implanted in or near the head
Also, TMS is self-pay for anxiety, insomnia, and OCD. Insurance only covers TMS for depression if multiple medications have failed.
Existing evidence to date suggests that patients who are less treatment-resistant respond better to TMS than those who are highly treatment-resistant. However, there is much yet to be learned about particular variables that may impact response to TMS. Researchers are presently conducting clinical studies to evaluate who will benefit most from TMS therapy. For example, there is a lot of interest in evaluating whether TMS with antidepressant medications or Spravato/ketamine is more effective than TMS alone.
TMS is one of the brain stimulation treatments for depression offered at Ascend Health Center. Before scheduling you for treatment, you must first be evaluated by our psychiatry team to determine if TMS would be safe and appropriate for you.
At Ascend, some patients are able to stop taking all of their meds and still don’t require additional treatment years later. About a fourth of patients don’t get better. The majority have depression that lifts by 50%, as documented on depression scales, and can reduce the number of anti-depressants they take. Insurance will reapprove TMS if the effects fade after six months, but we’ve never needed to do two series that close together.
Research and Studies
- Anderson, R. J., Hoy, K. E., Daskalakis, Z. J., & Fitzgerald, P. B. (2016). Repetitive transcranial magnetic stimulation for treatment resistant depression: Re-establishing connections. Clin Neurophysiol, 127(11), 3394-3405. doi:10.1016/j.clinph.2016.08.015
- Association, A. P. (2013). Diagnostic and Statistical Manual og Mental Disorders (DSM-5): American Psychiatric Association Publishing.
- Blumberger, D., Vila-Rodriguez, F., Thorpe, K. E., Feffer, K., Noda, Y., Giacobbe, P., . . . Downar, J. (2018). Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. The Lancet, April 28, 2018, volume 391, Issue 10131, p1683-1692.
- Chen, A. C., Oathes, D. J., Chang, C., Bradley, T., Zhou, Z. W., Williams, L. M., . . . Etkin, A. (2013). Causal interactions between fronto-parietal central executive and default-mode networks in humans. Proc Natl Acad Sci U S A, 110(49), 19944-19949. doi:10.1073/pnas.1311772110
- Downar, J., Blumberger, D. M., & Daskalakis, Z. J. (2016). The Neural Crossroads of Psychiatric Illness: An Emerging Target for Brain Stimulation. Trends Cogn Sci, 20(2), 107-120. doi:10.1016/j.tics.2015.10.007
- Lefaucheur, J. P., Andre-Obadia, N., Antal, A., Ayache, S. S., Baeken, C., Benninger, D. H., . . . Garcia-Larrea, L. (2014). Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol, 125(11), 2150-2206. doi:10.1016/j.clinph.2014.05.021
- Liston, C., Chen, A. C., Zebley, B. D., Drysdale, A. T., Gordon, R., Leuchter, B., . . . Dubin, M. J. (2014). Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry, 76(7), 517-526. doi:10.1016/j.biopsych.2014.01.023
- McClintock, S. M., Reti, I. M., Carpenter, L. L., McDonald, W. M., Dubin, M., Taylor, S. F., . . . Treatments. (2017). Consensus Recommendations for the Clinical Application of Repetitive Transcranial Magnetic Stimulation (rTMS) in the Treatment of Depression. J Clin Psychiatry. doi:10.4088/JCP.16cs10905
- Tik, M., Hoffmann, A., Sladky, R., Tomova, L., Hummer, A., Navarro de Lara, L., . . . Windischberger, C. (2017). Towards understanding rTMS mechanism of action: Stimulation of the DLPFC causes network-specific increase in functional connectivity. Neuroimage, 162, 289-296. doi:10.1016/j.neuroimage.2017.09.022
Take the First Step Towards Healing
Our team is dedicated to helping you find relief and regain control of your life.
today and begin your journey towards healing.